Preparation of Diazonium Salts
Preparation of Diazonium Salts: Overview
This Topic covers sub-topics such as Reaction of Diazotization
Important Questions on Preparation of Diazonium Salts
Identify Z in the change after diazotisation and sand Meyer's reaction.
Phenol reacts with benzenediazonium cation at pH 7.5 to give
Consider the following statements
Phenyl diazonium forms azodye with
aniline
phenol
N, N-dimethylaniline
anisole (methoxybenzene)
The reaction of ethyl p-aminobenzoate with and then with yields a compound (X), a crystalline ionic compound. Compound (X) when heated forms . The compound (Y) is
In which of the reaction formation of Diazonium salt takes place?
Predict respectively and in the following reactions
A compound on treatment with excess gives in presence of at . on treatment with and gives phenyl isocyanide. The diazonium salt from gives with ethanol. The incorrect option is:
When aniline is treated with a mixture of sodium nitrite and hypophosphorus acid, the product formed is
The ion formed by the reaction of and is
gas is liberated when reacts with which of the following compounds?
(A) (B) Urea
(C) (D)
When aniline is treated with sodium nitrite and hydrochloric acid at , it gives
Which will not go for diazotization?
Aniline in a set of reactions yielded a product .
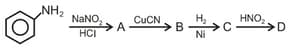
The structure of the product would be:
